Water, a major additive in the process of making tissue, also serves as a primary entry route for most of the microbes that colonize machines. Inadequate treatment can compromise machine cleanliness, machine runnability and as well as the quality of the finished product. Although all types of microbes can enter the mill through the incoming water, it is the filamentous bacteria, protozoa, algae, and microscopic worms that are generally referred to as fresh water contaminants since inadequately treated water is their primary route of entry.
The quality of incoming freshwater varies greatly, both regionally was well as seasonally. Rain, drought, seasonal turnover of water in lakes, mineral content and even pH must be considered when designing an effective freshwater treatment process. Treatment protocols that are suitable in a dry season may not be adequate during a high flood water period.
Properly treated water minimizes the formation of the biofilms (slime) that are a source of the sheet defects, holes and sheet breaks that lead to downtime. These biofilms often contain filamentous bacteria that can be extremely difficult to kill once they are in the nutrient-rich process. Filamentous bacteria form tangled strands that entrap other microorganisms, as well as wood fibers, fibrils and other functional additives (Figure 1). Many of filamentous bacteria have sheaths, structures that encase the cells in a protective layer, that impede penetration of both oxidizing and non-oxidizing biocides.
Figure 1: 400X phase-contrast photomicrograph of biofilm slime deposit. Debris particles are bound by the long thin strands of filamentous bacteria. Unicell bacteria can also be observed.
Water treatment
Treatment of incoming water requires more than merely maintaining a chlorine residual > 0.5 ppm measured using a DPD test. Oxidant treatment, water clarification, metals removal, pH considerations, contact time and storage times are critical factors to consider when treating water and achieving < 1 colony forming units/mL of microorganisms when plated on R2A or Standards Methods Agar.
Clarification
Engineers frequently underestimate how critical water clarification is in producing acceptable water for use on a tissue machine. Particulates bring metals, minerals and dirt-debris into the process, which can clog filters and cause sludge to accumulate in low turbulence zones. Microbes, imbedded within the debris particles are protected from antimicrobials. In general, mills experience added machine deposit problems when the turbidity of incoming surface water increases.
Water turbidity is measured in nephelometric turbidity units (NTU). In surface water turbidity can increase during lake turnover seasons, with runoff during heavy rain or even during drought periods. Ideally the NTU after water clarification should be under 1 NTU, a level rarely seen in most mills due to lack of an adequate coagulation/flocculation and/or poorly functioning sand filters. The author knows of a mill where water clarification is so critical to production that they need to increase biocide use within process when the NTU goes above 0.12 NTU in the freshwater. Unfortunately, treated water turbidities in the range of 30-40+ NTU are not unusual.
In addition to NTU measurements, a quick test to examine water clarification requires placing a gauze pad over a freshwater hose located near the machine. In this test, water runs through the hose at a rate of approximately 4 liters per minute for an hour. The collected “dirt” can be seen in the example shown in Figure 2. A portion of residue filtrate is extracted with a pipet, placed on a slide with a coverslip and examined microscopically with a phase-contrast microscope. The microscopist reports any living cells, which could include motile unicell bacteria, intact fungi, motile protozoa or algae with visibly intact green chloroplasts. The presence of any of these indicates that the water treatment is inadequate. A photomicrograph of material from the gauze pad are shown in Figure 3.
Figure 2: Debris collected on gauze after freshwater treatment.
Figure 3: 400X phase-contrast photomicrograph of treated freshwater filtrate collected on a gauze pad. Note viable green algae.
Water pH and Contact Time
pH has a profound influence on oxidants. Oxidants frequently used to treat freshwater include hypochlorite, chlorine gas, chlorine dioxide and activated bromine. Hypochlorite is often used because it is inexpensive, relatively easy to handle and effective as long as there is sufficient contact time to produce kill. To be effective the chlorine demand must be met and there must be sufficient oxidant to produce a measurable excess, which is the chlorine residual. Chlorine, bromine and other non-stabilized oxidants used for water treatment are corrosive at high concentrations. They also can damage felts, tinting dyes, wet strength, or other polymeric additives. This is why non-stabilized oxidants, measured as free-chlorine, are typically kept to 0.5-0.8 ppm in fresh water.
Kill is not instantaneous at these oxidant concentrations. In most cases, 30 minutes of contact time is sufficient with unionized HOCl at pH 6 (Figure 4). As the pH increases, the hypochlorite dissociates to OCl-, which is the inonized form of the oxidant. While still biocidal, it is slower-acting. A Canadian mill with pH 8 incoming raw water changed their oxidant addition point that had provided 90 minutes of contact time to an addition point with only 30 minutes. This resulted in massive filamentous stringers around the machine. Using chlorine or hypochlorite may be acceptable for higher pH water if the storage tanks are large enough to provide the extended contact time, which is rare. In mills with incoming water at pH 7 or above, activated bromine, HOBr, is recommended.
Figure 4: Dissociation of HOCl and HOBr as a function of pH.
A few mills have extended contact times of many hours due to oversized freshwater storage tanks. Sunlight and long storage times allow the oxidants to dissipate. Signs of growth can include the green algae seen on the walls of the storage tank in Figure 5 or even floating mats of algae. In situations like this the mill could consider a second chlorine or bromine addition point or the use of a stabilized oxidant such as monochloramine or hydantoin chemistry could be considered as an alternative antimicrobial oxidant. Stabilized oxidants have slower reaction times but greater persistence, which is why they are excellent for treating whitewater and thin stock within the machine.
Chest configurations
Ideally there is one entry point where all incoming water is treated with a biocidal oxidant and one exit point where water leaves for the machine. This configuration is more likely in newer mills. The older the mill the more likely that there will be two or more sources of water entering the storage tank or that the tanks will have channeling due to underwater barriers, treated water exits near entry points, stagnant areas, or other configurations that allows untreated water to exit to the machine. Some mills have “extra” water stored in tanks for emergency or fire use that does not maintain oxidant residuals to suppress microbial growth. This water is often accessed after a boil out and can reinoculated the process waters.
Figure 5: Wall of treated water storage chest showing growth of algae.
Metals
Iron and manganese are two metals that are utilized by certain types of bacteria. Filamentous bacteria such as Sphaerotilus deposit iron and Leptothrix deposit manganese. Ideally these metals are removed during the clarification stage. Oxidized iron and manganese can form sludge that clogs filters and narrows pipes. Sloughing of these deposits will form sheet defects.
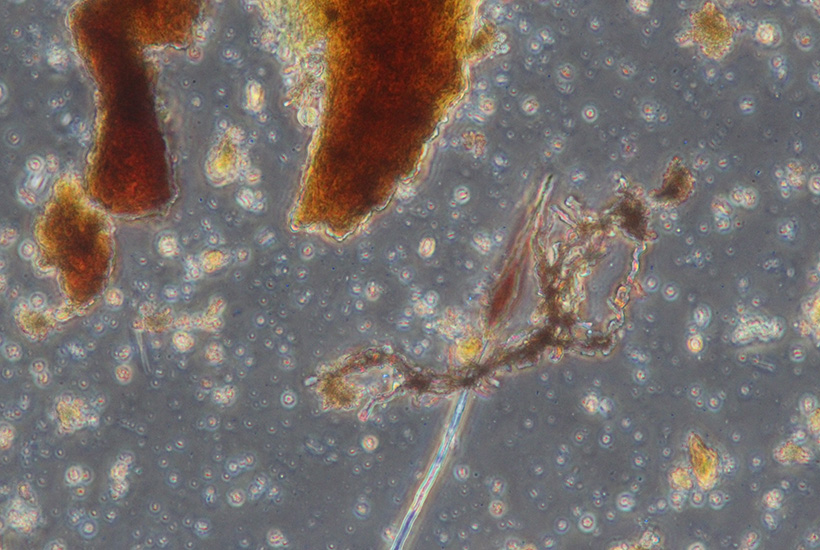 Figure 6: 400X magnification phase-contrast photomicrograph. Filamentous with iron encrustation. Numerous unicell bacteria also present.
Figure 6: 400X magnification phase-contrast photomicrograph. Filamentous with iron encrustation. Numerous unicell bacteria also present.
False positives
Manganese is also responsible for false positive reactions with the DPD test that measures oxidant residuals. According to HACH, 0.006 ppm Mn will give a false positive for oxidant showing 0.02 ppm chlorine using the low range DPD method. Freshwater containing 0.1 ppm Mn will add 0.33 ppm “chlorine” to the total residual, which means that oxidant residuals will be overestimated. Suspect manganese interference when the oxidant residuals are in the target range while the bacterial counts remain elevated and the ORP is lower than expected. Water chemistry tests will determine the manganese concentration. If it is present alternate DPD or other test methods must be utilized to determine the actual “chlorine” residual.
In conclusion
Microbes thrive in papermaking processes. The warm waters, nutrients from wood sugars, fibers, starch, defoamers, as well as carry over from recycle streams, all help provide an environment that allows a wide variety microorganism to flourish. Like a weed is a flower in the wrong place, the microbes causing problems in the machine are the same microorganisms that beneficially remove biochemical oxygen demand (BOD) in the wastewater treatment process. Unfortunately, their uncontrolled growth within the machine process causes costly downtime for wash ups and boilouts.
Rain, seasonal turnover of water in lakes, pH, and the presence of iron or manganese must be considered when designing an effective freshwater treatment process. High quality incoming freshwater helps minimize microbial growth within the process. Water is the largest volume additive in papermaking. Producing high quality incoming treated water minimizes biofilm formation. In turn, this helps reduce quality loss due to sheet defects, holes or the sheet breaks that lead to downtime.
Linda Robertson
International Microbial Associates
--------------------------------------------
Standard Methods: 4500-Cl G https://www.nemi.gov/methods/method_summary/7431/ Massa, S., Caruso, M., Trovatelli, F. et al. World Journal of Microbiology and Biotechnology (1998) 14: 727. https://doi.org/10.1023/A:1008893627877
B. Urtz Combined halogens: new products to combat an old problem, TAPPI Solutions!, Online Exclusives, March 2003 https://imisrise.tappi.org/TAPPI/Products/03/MAR/03MAROE02.aspx
W. L. VAN VEEN, et al. Microbiological Reviews (1978) 42: p. 329-356.
HACH Test Methods 2018 TE3395 https://support.hach.com/app/answers/answer_view/a_id/1003511/requestLocale/en_US/track/AvOh9worDv8S~QznGvQe~yL1TPsqsy75Mv_g~zj~PP9p

